Medical Device Recall Approvals
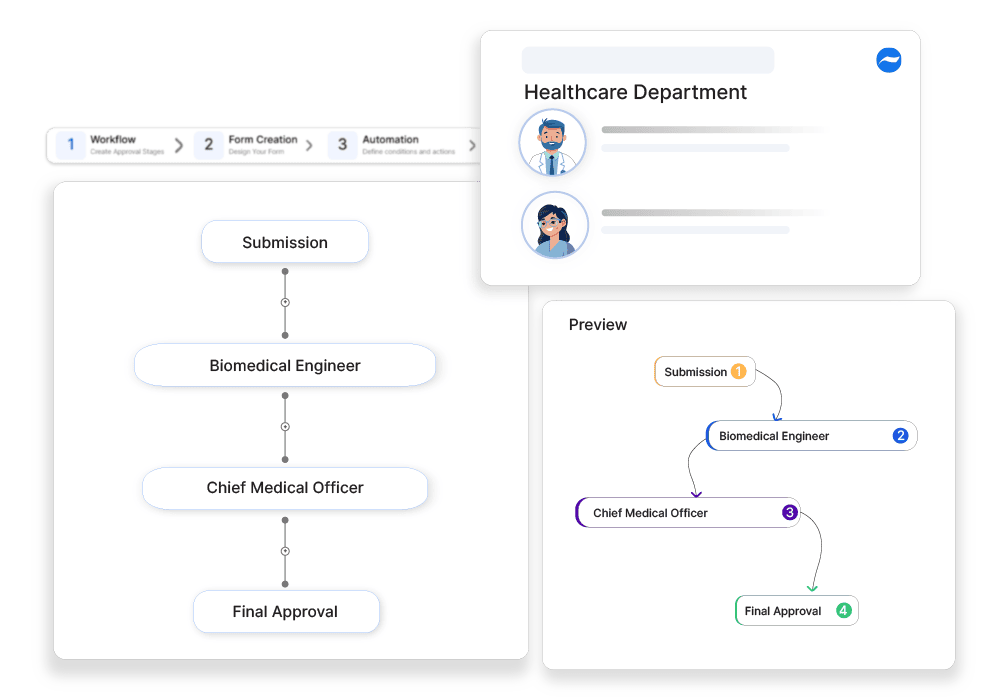
Why automate?
How Cflow Can Help Automate the Process:
Automated Approval Workflow:
Cflow automates the entire approval workflow for medical device recalls, ensuring that each recall is routed through the necessary stages for evaluation and approval. This reduces manual intervention and accelerates the recall process.
Real-time Tracking and Notifications:
Cflow provides real-time tracking of recall approvals, allowing stakeholders to monitor the status of each recall. Automated notifications are sent to relevant personnel, ensuring timely action and reducing the risk of delays.
Comprehensive Documentation and Compliance:
Cflow maintains detailed records of all recall approvals, including submission details, evaluation actions, and communication logs. This comprehensive documentation supports regulatory compliance and provides valuable insights for future reference.
Integration with Inventory Management Systems:
Cflow can be integrated with existing inventory management systems, allowing for seamless coordination of recall efforts and ensuring that affected devices are promptly identified and removed from use.
Frequently Asked Questions
What triggers a medical device recall?
Recalls are triggered by safety concerns, manufacturing defects, or regulatory non-compliance.
Who is responsible for initiating a recall?
The manufacturer, regulatory authorities (FDA, EMA), or healthcare providers can initiate recalls.
What are the key steps in managing a medical device recall?
Identification of affected devices, notification to users, corrective actions, and regulatory reporting.