Clinical Trial Participation Approvals
Enhance the clinical trial participation approval process with Cflow’s automated workflows.
Why automate?
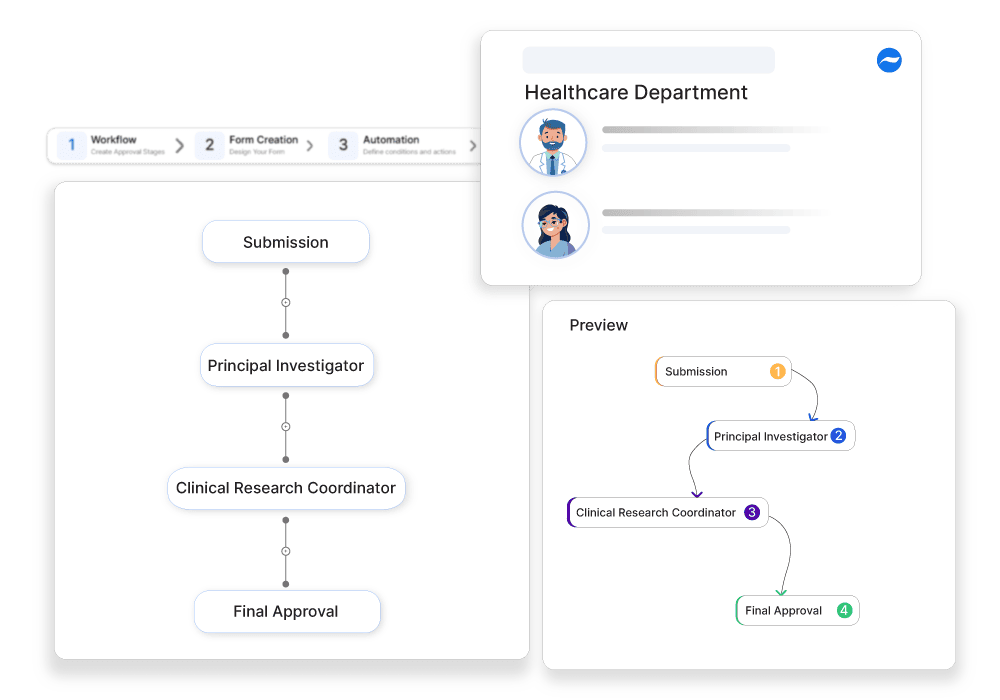
Clinical trials are a part of healthcare research and development that test the viability of the medication introduced newly, before making it available for public consumption in the market. However, trials can only be conducted on people who are interested, eligible based on certain criteria, and consent to the possible risks. Through this process, the medical history of the eligible person is reviewed, consent is obtained, and coordinated with researchers, ethics committees, and other regulatory bodies. To manage the complete process, the clinical trial participation approval process is used.
The traditional way of handling the trial participation approval process is manual and paper-based. This often leads to errors and multiple rounds of approvals, consuming a longer time to get the requests approved. This will delay patient enrollment and create compliance issues.
This is why automating the clinical trial participation approval process is ideal. This will help streamline the different tasks and tackle the challenges that put down the efficiency of the process. From the request creation to the final approval, the tool offers auto-verification, compliance checks, routing, and approval. This enhances the accuracy of approving eligible people for clinical trials.
How Cflow Can Help Automate the Process
Streamlined Request Submission
Cflow is a centralized platform that streamlines the collection and routing to efficiency. Requests are submitted through customizable forms that effectively capture all the necessary details to filter them for final approval.
Automated Verification
Cflow is a robust tool that auto-verifies patient eligibility and obtains consent from them, reducing the burden on administrative staff and the speed up the process.
Real-Time Communication
Cflow is a platform is effectively facilitates communication and collaboration between researchers, regulatory bodies, and patients. They will receive timely updates regarding the advancements.
Secure Document Management
Cflow ensures secure management and access to informed consent documents and other trial-related information, protecting sensitive patient data and ensuring compliance with regulatory requirements.
Transform & streamline business processes with cloud BPM & workflow automation software.
Join 100k + Users Who Are Already Using Cflow
“I’m really impressed with the support provided by Cflow. There has never been a time when they have kept me waiting. A product that is simple to use and a team that is smart and extremely fast are factors that help me feel reassured and confident.”

Ronald Tibay
Senior IT Manager @ NutriAsia, Inc
“The WFH environment during the COVID-19 pandemic made it clear how inefficient our processes were. Cflow allowed us to digitize paper forms with automated workflows. If you can envision a tool for an online workflow, it can be done in Cflow!”

Stephanie Duncan
Registrar @ Freed-Hardeman University
“We are extremely liking CFlow. So far any issues that we’ve had once we contacted support they were able to help us resolve the issue. This has helped us take a paper process and replace it, faster and more streamlined now for us.”

Bradley Wilkins
Director of Technology @Hazel Park School